Clinical Research
Struggling to Control High Triglycerides?
You may qualify for the ENTRUST study, which is testing an investigational research medication to find out if it can lower triglyceride (TG) levels. The ENTRUST study is a clinical research study for adults (age 22 and older) with very high TG levels (500 mg/dL* or higher), a condition known as severe hypertriglyceridemia (SHTG).
>> LEARN MORE
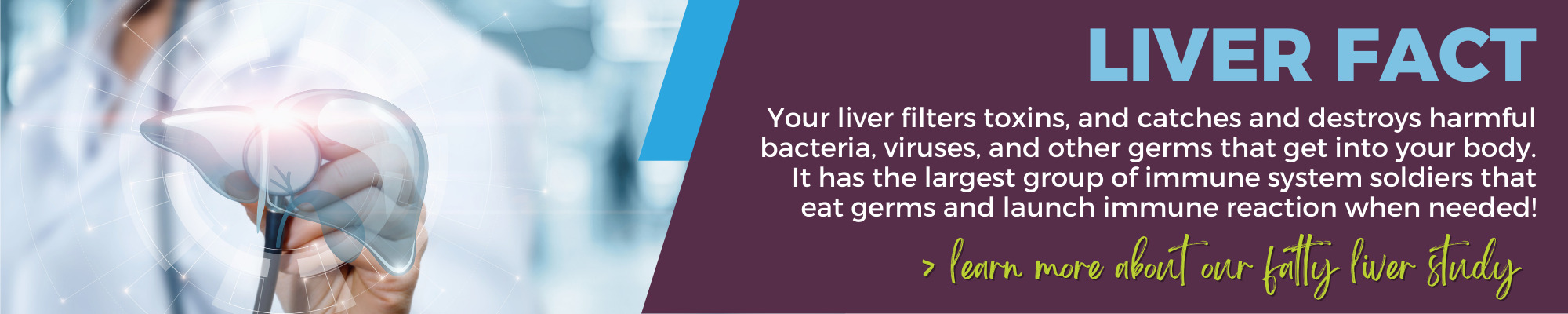
Do you have Fatty Liver Disease?
If you’ve been diagnosed with fatty liver disease, you may be eligible for one of our clinical studies. Nonalcoholic Steatohepatitis (NASH) is a form of fatty liver disease that is rapidly emerging as a leading cause of liver transplants in the US. As part of our study, you will get access to no-cost, non-invasive, at-home genetic testing, and if eligible, the opportunity to meet with a qualified genetic counselor. Get more information and see if you may be eligible.
>> PRINTABLE FLYER
Premier & Summit Clinical Research
Premier Medical is a part of the Summit Clinical Research network of experienced Non-Alcoholic Steatohepatitis (NASH) research sites. Summit is an Integrated Research Organization that brings together experienced research sites who have proven to be successful in the NASH research space. Recently, Summit interviewed Premier’s Director of Clinical Research Mary Kosinski about our partnership with Summit.
>> FULL ARTICLE
Is a Clinical Study Right for You?
Premier Medical partners with a variety of respected pharmaceutical companies to explore and test their newest medications, therapies, and diagnostic tools not available elsewhere. Learn more about the benefits and risks of clinical trial participation, and whether you may be a good candidate.
>> LEARN MORE
Clinical Research is a must for tomorrow’s break-through medications.
In fact, every prescription medication on the market has been studied through clinical research and monitored and approved by the Food and Drug Administration (FDA).
Premier Medical Group is helping to increase access to research studies, right here in the Clarksville area. We partner with a variety of respected pharmaceutical companies to explore and test their newest medications, therapies and diagnostic tools not available elsewhere. Participants in this cutting-edge research typically receive:
- Free study medication
- Free medical care
- Free medical procedures
- In addition, most studies monetarily compensate patients for their time and participation.